Fda 2011 Voluntary Ban on Medically Important Antibiotics in Feed
Positive practices put in place in animal agriculture to product both human and animal health.
In 2016 and 2017, the U.S. Food and Drug Administration (FDA) implemented new restrictions on how antibiotics can be used in food animal production. The updated veterinary feed directive (VFD), which was explained in Guidance Document 209 and 213, took effect January 1, 2017 and it changed how farmers could use antimicrobials that were deemed medically important to human medicine (but used in both human and animal medicine). The changes made focused in FDA Guidance Document 209 on a one-health approach, a key aspect of which is that antimicrobial drug use contributes to the emergence of drug resistant organisms and that these important drugs must be used judiciously in both animal and human medicine to slow the development of resistance. The biggest change for farmers was that, when utilizing certain feed-grade medications, farmers would need to follow a process that required them to first seek a directive (VFD) written by a veterinarian with whom the farmer had a valid veterinary-client-patient relationship (VCPR), in order to source antibiotics that would be delivered to the animals through the feed. This process provides a framework for all veterinarians who are involved in issuing these antimicrobials for use, and provides documentation requirements for the farmers using the antimicrobial, veterinarian issuing the VFD and feed mill processing the order. The purposes of the policy changes by FDA Guidance Document 209 were to promote the judicious use of antibiotics, protect public health and help to limit the development of antimicrobial resistance.
It is important to note that the United States is not the first county to incorporate stricter regulations regarding use of antibiotics in livestock, in the 1990's the European Union made the decision to phase out the use of antibiotics as growth promoters. This policy is similar to one of the changes to VFD regulations that US farmers incorporated over the last year, however Denmark further followed with a full voluntary ban in 1998, and that fully integrated in 2000. The overarching goal of the Danish regulations was to work towards a decrease of antibiotic resistance by reducing the use of antibiotics in human and animal health. Evaluation and summary data found through the DANMAP have shown an overall reduction with the use of antimicrobials for animals decreased for the fourth consecutive year and has since 2013 been reduced by more than 16 tonnes. From 2016 to 2017, the antimicrobial consumption, according to DANMAP 2017, decreased by approximately 3%. Similarly, the use of antibiotics in human medicine has also seen a reduction where decreases in the past ten years were observed for all age groups (excluding the eldest > 80 years) and for both genders, as recorded in the DANMAP 2017.
It is important to know that use of antibiotics in humans and food animals is comparatively low in Denmark, a county that produces approximately the same amount of pork as the State of Iowa, when compared to EU and the rest of the world. Still, there does appear to be an increasing number of resistant organisms in farm animals and humans, as reported by the DANMAP 2017. For example, resistance profiles where taken from Salmonella isolates from Danish pigs with some of the isolates showing, resistance to tetracycline, ampicillin and sulfonamides. This indicates a steady increase since 2010, this was echoed in samples taken from the human isolates, according to DANMAP 2017. This information summarized from Denmark leads to a number of questions regarding implementation of antibiotic use guidelines in the United States and what the unintended effects of this may be on production agriculture at a farm level.
It is understood that the VFD regulations had impacts at the farm level, on production practices and management of health. To gain a better understanding of the direct impacts to farmer raising animals for food productions Michigan State University Extension led a nationwide survey to help determine what effect these new rules had across food animal species and across farms on a national level. Farmer input was solicited and responses requested for survey questions that covered five areas: antibiotic use, animal morbidity and mortality, management, relationship with their veterinarian, and economics.
Survey responses were collected from farmers in 48 states and represented beef, dairy, sheep, goats, swine, poultry and other minor species. While data analysis is still ongoing, several consistent themes have emerged after the initial review of the data and responses. One theme is that some unintended economic impacts have occurred on farms because of the new VFD regulations, that is supported by producer comments such as, "The biggest change has been how much I have to pay the vet for treatment of my herd. It has increased the cost for production and for people who actually limited antibiotic usage before the regulation, the treatment has not changed, just the cost."
Other untended consequences of VFD compliance reported in the survey relate to animal health. When looking at animal morbidity and mortality some producers indicate that they see more animal sickness, have limitations on the availability of product to treat animals and are frustrated with the timeframe in which it takes them to source these products with VFD regulations, "While the VFD doesn't majorly affect my practices on a regular basis, it does limit the variety of options available to treat ailments and especially help supporting newborns, which can be frustrating." Farmers also indicted that sourcing VFD friendly businesses to support their operations can be challenging, stating "My veterinarian refuses to write a VFD. I have no other veterinarians in my area" and "It has been difficult finding feed suppliers in my area who are willing to carry VFD products. I have had to go without or pay much higher prices because of added shipping costs and additional veterinary costs."
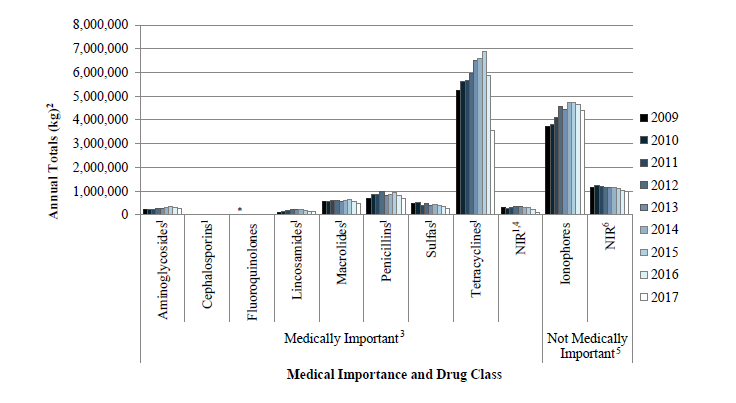
While there are some challenges to the ways that farmers have had to implement the VFD regulations, there are also positive impacts that these changes have created. For example, it appears that the critical goal of reducing farm use of medically important antibiotics is being achieved, thanks to the commitment of farmers to comply with VFD guidelines. Findings of medically important antimicrobials recently reported by the FDA indicate that sales and distribution of medically important antibiotics approved for use in livestock (all species combined) declined by 33% between the years 2016 and 2017, and by 43% since 2015. Summarized data on antimicrobial sales from 2009-2017 can be seen in figure one. Although the of the end use of these products cannot be adequately determined, it is assumed that with decrease of sales, decrease of use in food animal production use has occurred.
These results, reported by manufactures/distributors of the products, are consistent with data from the Michigan State University Extension survey results. Also, overall, the survey results indicate that communication with farm veterinarians and the use of vaccines have increased. This is supported by comments from farmers, including "VFD actually has helped us to find more preventive opportunities." This finding, from the National Pork Board, is highly encouraging, because strengthening the link between farmers and the veterinarians they work with should further improve America's farmers achieve their objective of protecting antibiotics for future use in humans and animals (4).
Further work, including data analysis and a determination of areas which may benefit from follow-up will be completed. Using the information gathered, Michigan State University Extension will be able to further support the One Health antimicrobial stewardship approach, by sharing the positive practices put in place in agriculture to protect both human and animal health.
Source: https://www.canr.msu.edu/news/veterinary-feed-directive-year-one-in-review
0 Response to "Fda 2011 Voluntary Ban on Medically Important Antibiotics in Feed"
Post a Comment